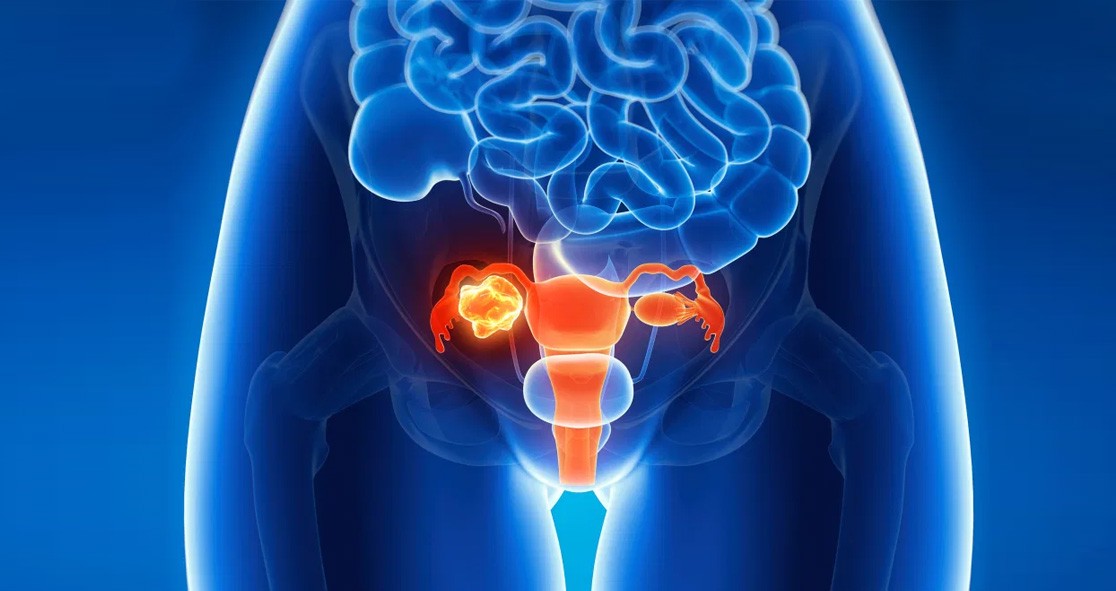
The U.S. Food and Drug Administration (FDA) has approved a fast track designation for STRO-002, a folate receptor α–targeting antibody-drug conjugate, for the treatment of advanced, platinum-resistant ovarian cancer.
Sutro Biopharma, the company that develops the drug, said the FDA approved the use of STRO-002 for the treatment of patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who have previously undergone treatment with 1 to 3 lines of systemic therapy, according to Cancer Network.
The drug is currently being explored in phase 1 trials for the treatment of endometrial and ovarian cancers.
Dr. Arturo Molina, Chief Medical Officer at Sutro Biopharma, said, “We are pleased with the FDA’s decision to grant fast track designation for STRO-002 and welcome the opportunity to have more frequent interactions with the agency.”
“We continue to be enthused by the potential of the STRO-002 program, which has shown encouraging preliminary activity and tolerability in our phase 1 dose-escalation study in ovarian cancer, and plan to continue to work with the FDA to potentially accelerate our clinical and regulatory efforts,” he added.
Dr. WendelNaumann, the trial’s principal investigator, said, “The preliminary evidence of anti-tumor activity we observed is encouraging, particularly in this heavily pre-treated patient population.”
“With limited therapeutic options for these patients, we are excited to continue to advance this clinical program to further investigate its therapeutic potential,” explained Dr. Naumann, a gynecologic oncologist at Levine Cancer Institute.
The investigators noted that STRO-002 was generally well-tolerated, with patients primarily experiencing low-grade adverse effects, such as fatigue, neutropenia, arthralgia, diarrhea, peripheral neuropathy, and myalgia.
There was one case of grade 4 neutropenia, but the investigators noted that all cases of neutropenia were reversible within a week.
Bill Newell, Chief Executive Officer at Sutro Biopharma, said in a statement, “Receiving Fast Track designation is an important recognition for STRO-002 as a potentially best-in-class FolRα ADC for women with ovarian cancer.
We look forward to further collaboration with the FDA to bring this potentially important therapeutic option to women in advanced stages of their disease with limited treatment options.”