The U.S. Food and Drug Administration (FDA) has granted “Priority Review” to dupilumab for the treatment of moderate to severe atopic dermatitis (AD) in children between the ages of 6 and 11.
Priority Review is one of the FDA’s programs to hasten the review process for drugs expected to have a great impact on the treatment of a disease.
Patients with moderate to severe AD can experience uncontrolled, persistent symptoms such as skin lesions, intense itching, skin dryness, skin cracking, redness, crusting, and oozing.
Dupilumab inhibits the key drivers of the type 2 inflammation that plays a key role in AD and other conditions.
According to recent clinical trials, children who received dupilumab along with topical corticosteroids experienced a significant improvement.
Common side effects of dupilumab and topical corticosteroids included nasopharyngitis, conjunctivitis, and reactions around the injection site.
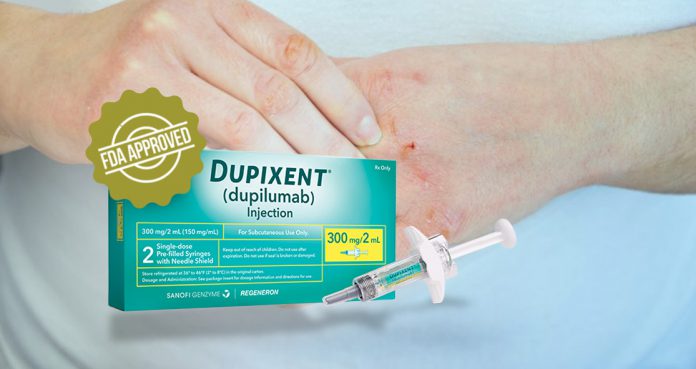
Developed by Regeneron and Sanofi, dupilumab is sold under the brand name Dupixent.
The FDA approved Dupixent for atopic dermatitis in people between the ages of 12 and 17 in March 2019 and the European Union (EU) approved the drug in August 2019.
The drug is also advised alongside other asthma medicines as maintenance therapy of moderate to severe eosinophilic. It is also advised along with oral steroids for asthma in patients aged 12 and above. The FDA will take the decision on this BLA in late May 2020.