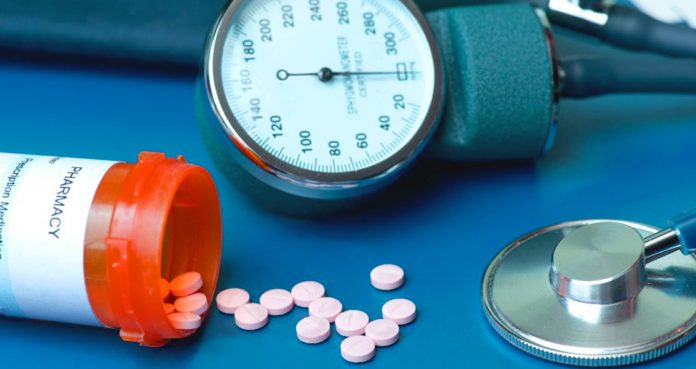
High blood pressure drugs containing valsartan, losartan and irbesartan manufactured by various companies have been recalled since July because they were found to contain ingredients that impose cancer risk. Valsartan, losartan, and irbesartan belong to angiotensin II receptor blockers (ARBs).
The U.S. Food and Drug Administration (FDA) keeps a regular update on the list of the drugs that have been recalled.
However, in this particular case, it is actually not a recall. An online pharmacy called Valisure said that it has tested every lot of medicine it dispenses. The tests included FDA standard assays and some proprietary analytical technologies. The pharmacy found a compound called N-Dimethylformamide (DMF) in a specific lot of valsartan.
The online pharmacy shared these findings with the FDA in a citizen petition on June 13. Valisure said it did so to intimate the FDA and requested the agency to take action.
DMF is a compound that can cause cancer, liver damage, and other health issues, according to the CDC. The chemical is used in production of some dyes, textile pigments, printing materials, and paint solvents and coatings.
FDA representative Jeremy Kahn wrote in an email, “Generally, the FDA will not comment on third-party research but evaluates it as a part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health. The FDA will review the Citizen Petitions and respond directly to the petitioner.”
Kahn added, “It is important to note that the amounts of DMF being reported are more than 100 items less than those determined by international standards as the level of concern to patients.”
The high blood pressure drugs that have been recalled have been tainted with a few impurities including N-