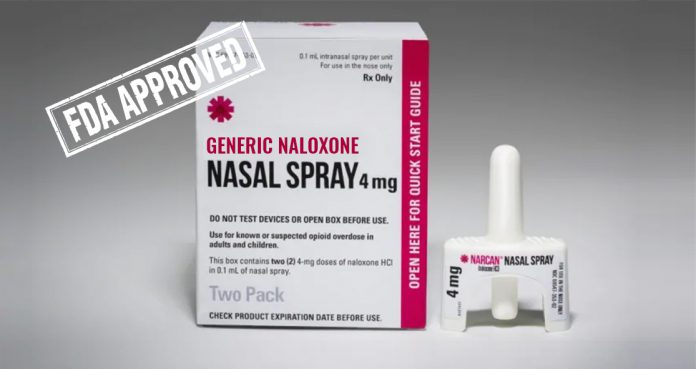
On Friday, the U.S. Food and Drug Administration (FDA) announced the approval of a generic version of naloxone nasal spray, a drug that is used as an antidote for fatal opioid overdoses.
Teva Pharmaceuticals, an Israeli-based company that specifically manufactures generic drugs, will provide generic naloxone in the form of nasal spray for use by anyone who has an opioid overdose, irrespective of their medical training.
Deputy center director for regulatory programs in the FDA Douglas Throckmorton said, “In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible. In addition to this approval of the first generic naloxone nasal spray, moving forward we will prioritize our review of generic drug applications for naloxone.”
Naloxone went off patent in 1980; however, various drug manufacturers have patented and received FDA approval for different versions of the drug.
Teva’s generic naloxone nasal spray got the FDA approval for community use with no specific medical training.
Last month, state health officials encouraged residents to carry opioid-overdose antidote naloxone.
Branded versions of naloxone are invariably more expensive than its generic version would be. And the higher drug price has limited the ability to easily stock the supply.
For instance, Evzio now costs around $4,000 per every two-pack, which was originally only $575 when it was approved by the FDA in 2014. Faced with the high drug price and bad publicity, Evzio manufacturer Kaleo announced last December that it would provide a generic version of Evzio in 2019 at a retail cost of $178.
Narcan, on the other hand, is much cheaper than Evzio, which is $130 for a two-pack. However, it is believed that Teva’s generic naloxone would be less expensive.
Teva has not yet issued any statement on the product availability or the price at this point. In addition to approving a generic version of naloxone, the drug regulatory body also said it would work with companies to come up with an over-the-counter version of naloxone, something that opioid policy experts and public health have long advocated for.