The U.S. Food and Drug Administration (FDA) has granted accelerated and emergency approval for a saliva test that could detect COVID-19, the disease caused by the novel coronavirus.
The new approach uses saliva as the primary test biomaterial to diagnose COVID-19.
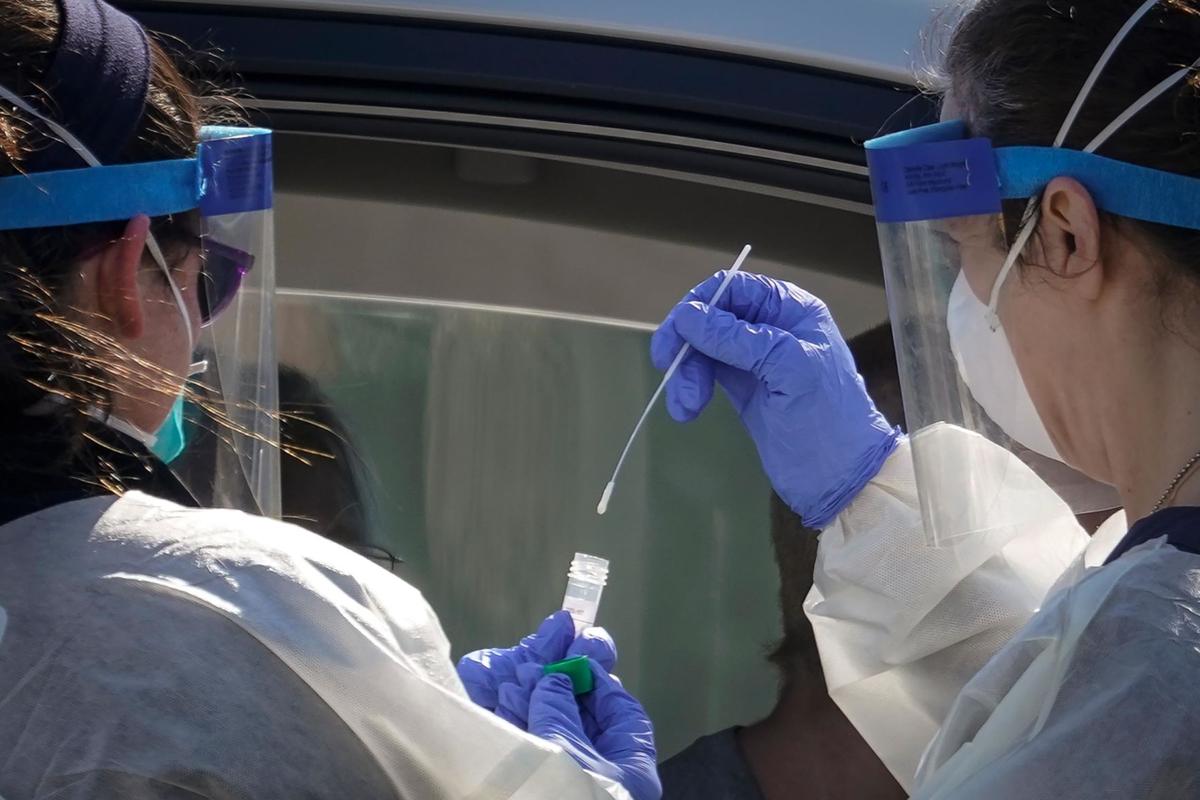
According to the FDA, “the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians.”
Researchers at Rutgers University developed the saliva collection method along with Spectrum Solutions and Accurate Diagnostic Labs.
The collection of saliva specimens is only limited to patients who have typical COVID-19 symptoms. It should be performed under a health care setting by a trained clinician.
The samples are then transported for RNA extraction, which are then tested within 48 hours of collection.
All of the saliva samples collected from 60 patients were in agreement with the presence of new coronavirus.
Dr. Matthew Cheng of McGill University, Montreal, who was not involved in the testing, said, “If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing.”
“Also, it may be easy enough for patients to do saliva self-collection at home,” he added. “However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [CDC] for respiratory virus testing.”
Chief Operating Officer of RUCDR Infinite Biologics Andrew Brooks said the new saliva collection method enables doctors to preserve personal protective equipment (PPE) for use in patient care instead of testing.
“We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” Brooks said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The saliva tests are now available to the RWJBarnabas Health network, West Orange, N.J., which has collaborated with Rutgers University. The article originally appeared on MDedge.com.